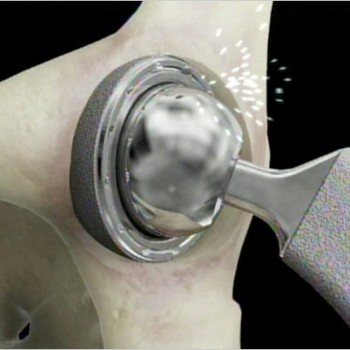
Metal on Metal Implant News
The European equivalent of the US FDA, the Medicines and Healthcare Products Regulatory Agency [MHRA], recently updated their recommendations for physician management and follow up visits for patients with metal-on-metal implants. Patients should be followed yearly and monitored for complications [such as chromium toxicity and device failure].